Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Survey on XAI powered system for the early detection of Alzheimers using Graph Neural Networks
Authors: Muquitha Almas, Dr. Kusuma Mohanchandra, Mohammad Mashhood Alam, Ridda Ishtiyaq, Mamoon Shakeel, Sandeep Rathod
DOI Link: https://doi.org/10.22214/ijraset.2024.59479
Certificate: View Certificate
Abstract
In light of recent advancements in eXplainable AI and graph neural networks, our survey paper seeks to analyze their potential in detecting Alzheimer’s disease through EEG datasets. We explore the various EEG datasets available for Alzheimer’s detection, discussing their unique characteristics and sources. We navigate through the landscape of classification methods weighing their pros and cons, and the challenges they present. We then explore attention to graph neural networks (GNNs), assessing their feasibility in Alzheimer’s detection and the potential they hold .We then explore functional connectivity measures and signal processing techniques that can be harnessed to create graphs, offering an in-depth analysis of their appli- cation. Lastly, we tackle the topic of explainability in GNNs, discussing how it can be implemented and evaluated in the context of Alzheimer’s detection. In paper we aim to shed light on the exciting possibilities of applying GNNs in Alzheimer’s detection
Introduction
I. INTRODUCTION
Alzheimer’s disease is a neurological disorder that impacts a significant population worldwide. It is characterised by the accumulation of protein material in the brain,which leads to the progressive and irreversible loss of cognitive and functional abilities.Two primary methods for detecting Alzheimer’s are EEG scan data and MRI scan data.This paper includes an ex- haustive survey on predicting AD or Alzheimer’s disease using Electroencephalography (EEG).Researchers have extensively explored employing EEG as a diagnostic tool for Alzheimer’s disease(AD) by examining the EEG signals of individuals with AD solely in comparison to those of healthy subjects.
EEG is a useful tool in Alzheimer’s disease research because it provides real-time and non-invasive measurements of brain activity.Compared to fMRI, EEG is less invasive and more affordable and has a much higher temporal resolution. . The primary objective of an EEG is to gather the collective electrical impulses generated by countless neurons within the cerebral cortex. The signal recorded from an electrode is commonly identified as a channel. EEG acquisition lacks a uniform standard procedure, varying in the number and placement of electrodes used across different methodologies. Most procedures are based on the measurement system that was proposed by H. H. Jasper [1], which is also known as the 10-20 montage.
The first section of the literature survey explains the various sources and the characteristics of the available EEG datasets for Alzheimer’s disease detection. Different types of methods developed for Alzheimer’s detection are covered in the second section. The third section summarises the feasibility of Graph Neural Networks in the task of detecting Alzheimer’s. The fourth section presents a survey on which functional connec- tivity measures should be used in creating the EEGraph. The last section focuses on the integration of explainability within the model, aiming to facilitate the interpretation of results generated.
II. LITERATURE SURVEY
The primary objective of this research is to emphasize the work done in Alzheimer’s detection through the analysis of EEG data. To achieve this, we undertake a thorough survey centered on Alzheimer’s detection, addressing key research questions (RQs).
- RQ1: What are the characteristics and sources of the available EEG datasets for Alzheimer’s disease detection.
- RQ2: What are the different methods of classification and challenges associated with them?
- RQ3: What is the feasibility of graph neural networks for Alzheimer’s disease detection?
- RQ4: What are the functional connectivity measures that can be used in creating graphs?
- RQ5: How can the explainability of GNNs be imple- mented and evaluated for Alzheimer’s disease detection?
A. Dataset
This section will cover the availability and usability of various EEG datasets which can be suitable for the detection of Alzheimer’s disease.
- RQ1: What are the characteristics and sources of the available EEG datasets for Alzheimer’s disease detection?
Dominik Klepl, et al [2], used an EEG dataset encompassing 20 individuals who were diagnosed with Alzheimer’s disease (AD) and 20 healthy control participants under the age of 70 . A this dataset was also employed in Blackburn’s study [3].The AD individuals were carefully selected from the memory clinic at Sheffield Teaching Hospital. All AD subjects had been diagnosed between one month and two years prior to recording, and the condition was present in mild to moderate stages. Ag/AgCL electrodes and an XLTEK 128-channel headbox were used to record the EEG data. A modified 10-10 electrode placement technique that overlapped with the 10-20 international standard sampled at 2 kHz was used. For the duration of each 30-minute recording session, participants were told to unwind and not concentrate on any particular ideas.There were two-minute long portions in these recordings where individuals’ eyes were closed (interspersed with equally lengthy segments while their eyes were open, which were not included in this study). The EEG recordings were made by a group of neurologists from the Department of Neurology at the AHEPA General Hospital in Thessaloniki. A Nihon Kohden EEG 2100 clinical device was used to enable the recordings. It has 19 scalp electrodes that adhere to the 10-20 international standard, and, per the device’s handbook, two reference electrodes that are inserted on mastoids to check for impedance. To be included in the dataset, the EEG data were first stored in the.eeg format and then converted into the BIDS-compatible.set format. Notably, some EEG recordings showed abnormalities related to eye movement even though the recordings were made while the subject was at rest and had closed eyes. Each subject had three artifact-free epochs of 12 seconds separated. The readings were examined by a skilled neuro physiologist on the XLTEK review station, which used the time-tracked video recording approach to produce 23 bipolar channels. [3].
Andreas Miltiadous.,et al [4] have provided an EEG dataset which comprises observations of 88 individuals eyes closed while they remained in the state of rest.Of them, thirty-nine had been identified to be healthy subjects (CN group), thirty-six were found to have been diagnosed with Alzheimer’s disease (AD group), and twenty-three had been identified with dementia of the frontotemporal region (FTD group). Using the worldwide Mini-Mental status Examination, cognitive and cognitive position was evaluated (MMSE). A low MMSE score indicates a greater degree of cognitive disorder. The MMSE rating can range from 0 to 30. If the disease’s length was measured in months, its median value is 25, and the IQR ranged between 24 to 28.5 months. Additionally, there were no reports of dementia-related illnesses related to AD groups. The mean MMSE was 17.75 for AD, 22.17 for FTD, and 30 for CN.
Dimas Avila Mart´?nez [5] has used a dataset in his work which possesses three classes: Alzheimer’s Disease, mild cognitive impairment, Healthy Control. EEG documents total of sixty-three for each subject class. The recording procedure was done using 19 electrodes at 256 Hz, based on the 10-20 template standard. The participants were watched for several minutes while at rest. That is no activities were carried out during the recording duration. The information captured contained parameters such as sampling rate, date of recording and data captured by the sensors is stored in a 19 dimensional array(one per electrode). The recordings followed the pattern of: patient id class.each of the recordings were divided into rectangular, non-overlapping windows which had a duration of 5 seconds i.e.,1280 samples these are also called epochs. Since the length of the recordings varied from patient to patient, the dataset procured after splitting resulted in a very unbalanced dataset which had more than two times more HC samples than AD samples Table 1 describes the distribution of the samples:
TABLE I
Dataset Sample Distribution [5]
|
Class |
Number of Epocs |
Relative Percentage |
|
HC |
6461 |
47.34 % |
|
AD |
2756 |
20.19 % |
|
MCI |
4430 |
32.46 % |
The parent recording of each epoch was used to identify it. This means that every one of epochs created by one recording category which were identified as AD would also be marked as AD. To guarantee backward compatibility, every period was saved as .mat file.
The naming pattern for the records was followed, but an index was appended at the end of the name to differentiate between the epochs: patient id class epoch index. Each of the aforementioned classes possesses its own directory storing the dataset. Ultimately, a.csv file was created which contained the whole path of all files along with their labels, making it easier to navigate over the entire dataset.
B. Methods
Throughout our search, we have explored various methods for the detection of Alzheimer’s disease using an EEG dataset.
- RQ2: What are the different methods of Alzheimer’s detection?
A.Dhiya Al-Jumeily., [6] The parent recording of each epoch was used to identify it. This means that every one of epochs created by one recording category which were identified as AD would also be marked as AD. To guarantee backward compatibility, every period was saved as .mat file. The naming sequence for the records was followed, but an index was included at the end of the name to differentiate between the epochs: patient id class epoch index. Each of the aforementioned classes possesses its own directory storing the dataset. Ultimately, a.csv file was created which contained the whole path of all files along with their labels, making it easier to navigate over the entire dataset. The Mann-Whitney U test was used to analyse the experiment outcomes. This study aimed to demonstrate the importance of using the PCA approach to remove duplicate data from datasets in order to obtain more dependable findings.
The cross-correlation measure, as opposed to phase synchrony, demonstrated a larger difference in the synchronisation of patients with moderate Alzheimer’s disease (MiAD) and control subjects, according to the results.
In order to effectively capitalize on the advantages of unsupervised feature learning, B. Yilu Zhao [7] has developed a deep learning network for the interpretation of EEG data related to Alzheimer’s disease. The study included fifteen patients with a clinical diagnosis of Alzheimer’s disease and fifteen healthy participants.
Each person possesses sixteen electrodes. The time domain EEG data from each electrode is split into 40 data units based on the volume of data collected in a particular period. After being trained with 25 data units on each electrode individually, the deep learning network is evaluated using 15 data units to ascertain the accuracy on each electrode. Subsequently, SVM training is implemented and the learning outputs are ultimately averaged over 16 electrodes.After combining each person’s 16 electrodes, an accuracy of 92% was found.
The deep learning model on Alzheimer’s disease may be enhanced with the upcoming new data, space, and computing time by substituting the existing data with new data. By using increment learning, accuracy rose by 0.5%.
C. Khalil Alsharabi., [8] suggested utilizing a band-pass elliptic digital filter to eliminate disturbances and interference from the EEG dataset. The filtered signal can then be divided into its frequency bands using a technique known as the Discrete Wavelet Transform (DWT) in order to retrieve the parameters of EEG signals.
A number of signal parameters, including as variance, kurtosis, average energy, logarithmic band power, standard deviation, and variance, have been added to the DWT approach in order to generate feature vectors and improve diagnosis performance. Subsequently, nine machine learning techniques have been used to study the classification of EEG features into their respective classes: k-nearest neighbor (KNN), decision tree (DT), support vector machine (SVM), Na¨?ve bayes (NB), extreme learning machine (ELM), artificial neural network (ANN), random forests (RF), linear discriminant analysis (LDA), k-nearest neighbor (KNN) and quadratic discriminant analysis (QDA).
In the end, the efficacy of the various proposed machine learning techniques has been evaluated and compared by computing the sensitivity, specificity, overall diagnosis accuracy, and area under the receiver operating characteristic (ROC) curves and visualizing the ROC curves and confusion matrices for five classification problems. The findings showed that the KNN classifier achieved an area under the ROC curve of 100% and an average classification accuracy of 99.98%. The authors conclude that their proposed methods are a valuable supplement to existing resources for identifying putative biomarkers that may help with Alzheimer’s disease clinical diagnosis.
Recurrent neural networks (RNNs) and wavelet pre- processing were developed and trained by D. Petrosian A.A.., [9] to distinguish the EEGs of mild Alzheimer’s disease (AD) patients from those of age-matched control participants.The parieto-occipital channels of ten early AD patients and ten healthy controls were used to capture continuous EEGs of their closed eyelids during rest, along with their wavelet- filtered subbands. These recordings were then input into RNNs for training and testing.For left parietal channel level 4 high-pass wavelet sub bands, a three-layer RNN yielded the best training/testing outcomes.
After being trained on three recordings of AD and three recordings of controls, the resultant RNN performed well on the remaining controls and five out of seven AD patients. A performance of 80% sensitivity and 100% specificity was recorded by the model. Before its clinical diagnostic usefulness can be confirmed, the study suggested that the combined wavelet/RNN approach can be applied to bigger patient groups and used to analyse long-term continuous EEGs for early detection of AD.
C. Graph Neural Networks
This part investigates the viability of using graph neural networks to categorize Alzheimer’s disease (AD). Neural net- works specifically engineered to function on graph-structured data are called Graph Neural Networks (GNNs). GNNs are designed specifically for data that may be represented as graphs with edges connecting things (nodes).
- RQ3: What is the feasibility of graph neural networks for Alzheimer’s disease detection?
Ana M. Maitin et. al. [10]in their paper have presented an open source Python library called EEGraph that models electroencephalograms (EEGs) as graphs to analyze the con- nectivity between different brain areas. The library can export the graph as a NetworkX graph-like object or graphically visualize it. EEGraph has applications in the study of neurological diseases such as Parkinson’s or epilepsy.
The authors demon- strate the effectiveness of EEGraph by modeling EEGs from a publicly available dataset and analyzing the connectivity between different brain regions. With its extensive variety of connection metrics and several output possibilities, EEGraph is a user-friendly and highly effective tool with immediate applications in the fields of clinical and neurological research. In D. Klepl et al.’s implementation of GNNs [2], sensor- level EEG data is converted into FC brain diagrams using eight functional connectivity (FC) measures.
After GNN models are trained, the efficacy of the selected FC measures is compared. The GNN models perform significantly better than the other baseline models, as the authors show. Moreover, GNN outper- forms models trained with a preset graph based on the spatial separation between the EEG sensors when predicting brain graphs with FC values. But none of the FC metrics regularly outperforms the others.
The best convolutional neural network (CNN) achieves an accuracy of 84.7% and an area under the sensitivity-specificity curve (AUC) of 0.984, whereas the best baseline model achieves an AUC of 0.924.
D. Avilia [5] developed a method for using GNNs to analyze data by transforming unprocessed recordings into a graph representation. Understanding how a graph is put together is the first step in making one. This involves defining the nodes and their attributes, the edges that link the nodes, and the weights (or attributes) that are applied to the edges. The edge and the node features are described further in the next section. Pytorch Geometric (PyG), a library designed to work with graph-like data and enable the development of GNNs, is utilised to do this.
In Pytorch Geometric, The Data class defines graphs as objects, and these objects need four components.After con- structing the graphs, the next step is to create the models that will analyse them and carry out the classification opera- tion.The model which was used was GraphConvNetAttention that accepts a fully connected, unweighted graph as input; the network uses attention to learn the weights. TransformerConv was the graph attention convolution layer that performed the best. with an accuracy of 92.31 %, defined in [11].There were two attention heads .
Aruane M. Pineda et al., [12] in his study has introduced a novel approach utilizing quantile graphs (QGs) to analyze EEG data for classification purposes . QGs translate the correlation, amplitude, and frequency properties of a time series—like an AD patient’s EEG data—into a network’s topological charac- teristics. Five network measures showed which scalp channels gave the highest discrimination, allowing healthy people to be separated from AD sufferers. Results suggested that all AD patients exhibited clear disease symptoms, potentially in its late stage.
In conclusion, The collection of findings in this research confirms that the QG approach is a useful tool for complicated temporal pattern analysis, such as that shown in the EEGs of AD patients.The QG method effectively analyzes complex EEG signals, showing promise for AD diagnosis.
However, it’s important to note limitations, such as the absence of definitive AD diagnoses for subjects and the small sample size, which restricts generalizations. Further extensive research with larger datasets is essential to validate the QG method’s efficacy in early AD diagnosis, especially in patients with mild cognitive impairment.
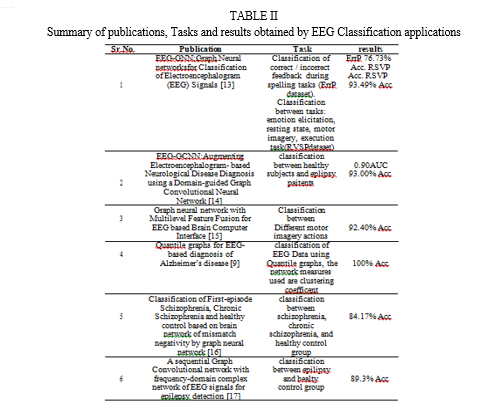
D. Functional Connectivity Measures
Eight popular functional connectivity measures—the phase lagged index (PLI), weighted phase lagged index (wPLI), phase locking value (PLV), mutual information (MI), ampli- tude envelope correlation (AEC), spectral coherence (coh), the imaginary part of coherency (iCOH), and the absolute value of Pearson’s correlation (corr)—are described and used in the paper [2].


It works by generating a simplified graph by removing or adding edges and nodes, aiming to approximate the behavior of the original GNN. It was the first approach to provide understandable explanations for predictions made by any GNN-based model on any graph-based machine learning task that was model-neutral overall. A restricted selection of node properties and a compact subgraph structure are identified by GNNExplainer as being essential to GNN’s prediction.This has been formulated as optimisation job that optimises the mutual information between the distribution of potential subgraph structures and a GNN’s prediction. Additionally, the explainer can produce succinct and coherent explanations for every instance in a class.Important graph structures and node properties were discovered to be identified using this technique, which outperforms baselines by an average of 17.1%. It also offers other advantages, including as interpretability, the visualisation of semantically significant structures, and the ability to identify mistakes made by flawed GNNs.
In order to generalize the parameterization of the explanation generation process and enable a more natural approach to explaining multiple instances collectively, Dongsheng et al [25] propose a parameterized explainer for GNNs. Compared to other approaches this approach has better generalization ability compared to the existing work. Experiments conducted on both synthetic and real-world graphs have shown significant graph topologies and node properties. The explanation accuracy of this technique surpasses that of its alternative baseline approaches by as much as 24%.
Conclusion
A significant possibility for classifying people with and without Alzheimer’s disease has been found through an exam- ination of EEG data. In this study we focused our efforts in ex- amination and analysis of diverse datasets. Within this paper, a comprehensive survey has been conducted on various method- ologies, including SVM (Support Vector Machines), Discrete Wavelet Transform, Recurrent Neural Networks (RNNs) and Graph Neural Networks. .It was noted that Graph Neural Networks (GNNs) exhibit potential for detecting Alzheimer’s disease.The top-performing GNN based models have achieved an impressive 0.984 AUC (Area Under Sensitivity-Specificity Curve) and demonstrated a 92% accuracy rate. Among the various graph attention convolution layers, the Transformer- Conv exhibited the most favorable performance, achieving an accuracy of 92.31%.From this survey we also find that the existing medical graph models lack explainability and hence their behaviour with real world data might not be reliable. Hence, we then focus our efforts on analyzing the various Graph Neural Network Explainer techniques to determine their useablility on medical graph neural network models.
References
[1] J. HH, “The ten-twenty electrode system of the international federation,”Electroenceph clin Neurophysiol, vol. 10, pp. 367–380, 1958. [2] D. Klepl, F. He, M. Wu, D. J. Blackburn, and P. Sarrigiannis, “Eeg-based graph neural network classification of alzheimer’s disease: An empirical evaluation of functional connectivity methods,” IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 30, pp. 2651–2660, 2022. [3] D. J. Blackburn, Y. Zhao, M. De Marco, S. M. Bell, F. He, H.-L. Wei, S. Lawrence, Z. C. Unwin, M. Blyth, J. Angel, et al., “A pilot study investigating a novel non-linear measure of eyes open versus eyes closed eeg synchronization in people with alzheimer’s disease and healthy controls,” Brain sciences, vol. 8, no. 7, p. 134, 2018. [4] A. Miltiadous, K. D. Tzimourta, T. Afrantou, P. Ioannidis, N. Grigori- adis, D. G. Tsalikakis, P. Angelidis, M. G. Tsipouras, E. Glavas, N. Gian- nakeas, et al., “A dataset of eeg recordings from: Alzheimer’s disease, frontotemporal dementia and healthy subjects,” OpenNeuro.[Dataset], 2023. [5] D. A´ vila Mart´?nez, “Graph neural networks for electroencephalogram analysis,” Master’s thesis, Universitat Polite`cnica de Catalunya, 2022. [6] D. Al-Jumeily, S. Iram, A. J. Hussain, V. Francois-Benois, and P. Fergus, “Early detection method of alzheimer’s disease using eeg signals,” in Intelligent Computing in Bioinformatics: 10th International Conference, ICIC 2014, Taiyuan, China, August 3-6, 2014. Proceedings 10, pp. 25–33, Springer, 2014. [7] Y. Zhao and L. He, “Deep learning in the eeg diagnosis of alzheimer’s disease,” in Computer Vision-ACCV 2014 Workshops: Singapore, Singa- pore, November 1-2, 2014, Revised Selected Papers, Part I 12, pp. 340–353, Springer, 2015. [8] K. AlSharabi, Y. B. Salamah, A. M. Abdurraqeeb, M. Aljalal, and F. A. Alturki, “Eeg signal processing for alzheimer’s disorders using discrete wavelet transform and machine learning approaches,” IEEE Access, vol. 10, pp. 89781–89797, 2022. [9] A. Petrosian, D. Prokhorov, W. Lajara-Nanson, and R. Schiffer, “Recur- rent neural network-based approach for early recognition of alzheimer’s disease in eeg,” Clinical Neurophysiology, vol. 112, no. 8, pp. 1378– 1387, 2001. [10] A. M. Maitin, A. Nogales, P. Chazarra, and A´ . J. Garc´?a-Tejedor, “Ee- graph: An open-source python library for modeling electroencephalo- grams using graphs,” Neurocomputing, vol. 519, pp. 127–134, 2023. [11] Y. Shi, Z. Huang, S. Feng, H. Zhong, W. Wang, and Y. Sun, “Masked label prediction: Unified message passing model for semi-supervised classification,” arXiv preprint arXiv:2009.03509, 2020. [12] A. M. Pineda, F. M. Ramos, L. E. Betting, and A. S. Campanharo, “Quantile graphs for eeg-based diagnosis of alzheimer’s disease,” Plos one, vol. 15, no. 6, p. e0231169, 2020. [13] A. Demir, T. Koike-Akino, Y. Wang, M. Haruna, and D. Erdogmus, “Eeg-gnn: Graph neural networks for classification of electroencephalo- gram (eeg) signals,” in 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), pp. 1061– 1067, IEEE, 2021. [14] N. Wagh and Y. Varatharajah, “Eeg-gcnn: Augmenting electroencephalogram-based neurological disease diagnosis using a domain-guided graph convolutional neural network,” in Machine Learning for Health, pp. 367–378, PMLR, 2020. [15] Y. Kwak, W.-J. Song, and S.-E. Kim, “Graph neural network with multilevel feature fusion for eeg based brain-computer interface,” in 2020 IEEE International Conference on Consumer Electronics-Asia (ICCE-Asia), pp. 1–3, IEEE, 2020. [16] Q. Chang, C. Li, Q. Tian, Q. Bo, J. Zhang, Y. Xiong, and C. Wang, “Classification of first-episode schizophrenia, chronic schizophrenia and healthy control based on brain network of mismatch negativity by graph neural network,” IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 29, pp. 1784–1794, 2021. [17] J. Wang, S. Liang, D. He, Y. Wang, Y. Wu, and Y. Zhang, “A sequential graph convolutional network with frequency-domain complex network of eeg signals for epilepsy detection,” in 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), pp. 785–792, IEEE, 2020. [18] M. M. Rahman, S. A. Fattah, et al., “Mental task classification scheme utilizing correlation coefficient extracted from interchannel intrinsic mode function,” BioMed research international, vol. 2017, 2017. [19] Pawan and R. Dhiman, “Electroencephalogram channel selection based on pearson correlation coefficient for motor imagery-brain-computer interface,” Measurement: Sensors, vol. 25, p. 100616, 2023. [20] G. Vecchiato, L. Astolfi, F. D. V. Fallani, J. Toppi, F. Aloise, F. Bez, D. Wei, W. Kong, J. Dai, F. Cincotti, et al., “On the use of eeg or meg brain imaging tools in neuromarketing research,” Computational intelligence and neuroscience, vol. 2011, 2011. [21] G. Varone, W. Boulila, M. L. Giudice, B. Benjdira, N. Mammone, C. Ieracitano, K. Dashtipour, S. Neri, S. Gasparini, F. C. Morabito, et al., “Signatures of brain network alteration in psychogenic non-epileptic seizures: A rest-eeg study based on power spectral density and phase lag index,” bioRxiv, pp. 2021–10, 2021. [22] U. Dutta, K. Sharma, R. A. Ganesan, et al., “Functional connectivity methods for eeg-based biometrics on a large, heterogeneous dataset,” arXiv preprint arXiv:2206.01475, 2022. [23] M. Abazid, N. Houmani, J. Boudy, B. Dorizzi, J. Mariani, and K. Kin- ugawa, “A comparative study of functional connectivity measures for brain network analysis in the context of ad detection with eeg,” Entropy, vol. 23, no. 11, 2021. [24] Z. Ying, D. Bourgeois, J. You, M. Zitnik, and J. Leskovec, “Gnnex- plainer: Generating explanations for graph neural networks,” Advances in neural information processing systems, vol. 32, 2019. [25] D. Luo, W. Cheng, D. Xu, W. Yu, B. Zong, H. Chen, and X. Zhang, “Parameterized explainer for graph neural network,” 2020.
Copyright
Copyright © 2024 Muquitha Almas, Dr. Kusuma Mohanchandra, Mohammad Mashhood Alam, Ridda Ishtiyaq, Mamoon Shakeel, Sandeep Rathod. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET59479
Publish Date : 2024-03-27
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

